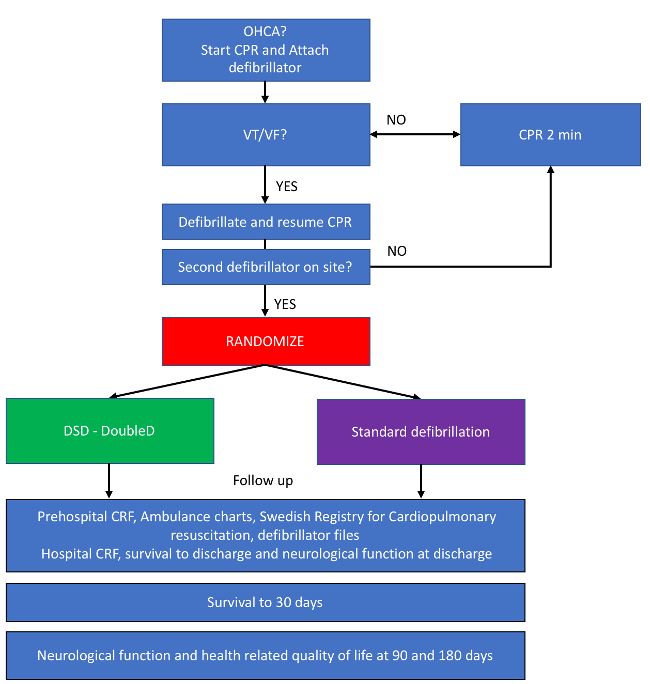
Main Trial
DoubleD-Trial is an academic, investigator-initiated, open-label randomized study.
The aim is to evaluate whether early double sequential defibrillation (DSD) improves survival compared with standard defibrillation in out-of-hospital cardiac arrest (OHCA) patients with an initial shockable rhythm who remain in cardiac arrest after at least one standard shock.
The trial is coordinated by the Centre for Resuscitation Science, Karolinska Institutet (sponsor).
Inclusion is performed by EMS units with two study defibrillators available (currently Corpuls3). Eligible patients are >18 years with VT/VF after at least one standard A-L defibrillation.
A pilot study (n=40) in West Sweden was completed in April 2025. The main trial will start recruitment on September 1, 2025.
Inclusion Criteria
✅ OHCA with a primary shockable rhythm and at least one defibrillation performed in standard A-L position
Exclusion Criteria
❌ Age < 18 years
❌ Obvious pregnancy
❌ Known preexisting Do Not Attempt Resuscitation order
Control group
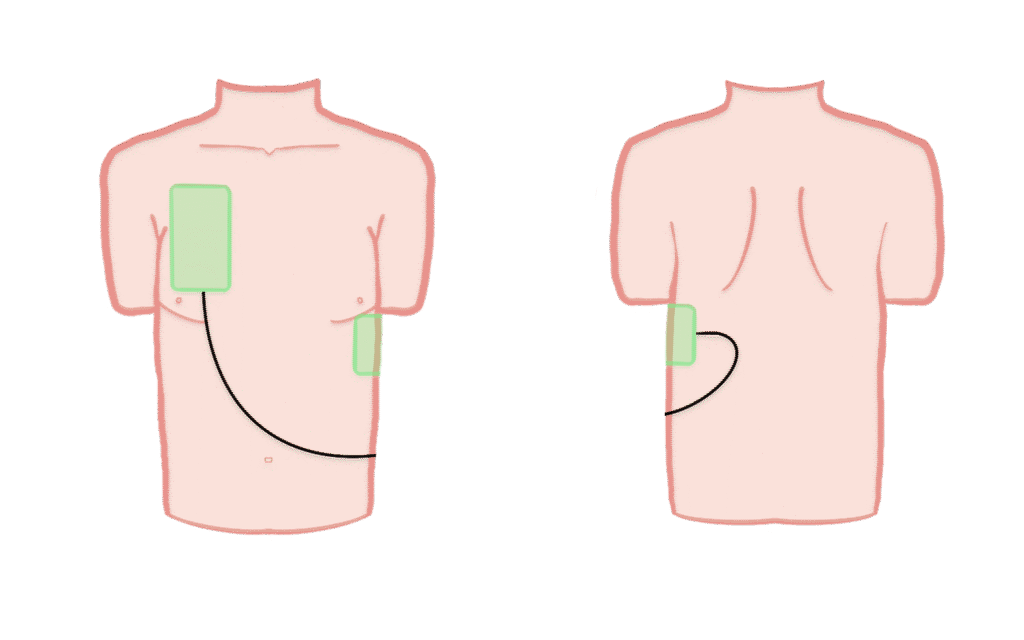
Patients in the control group receive standard Advanced Life Support. Defibrillation is performed with one defibrillator in the standard A-L position and continued until ROSC, termination of resuscitation, or hospital arrival.
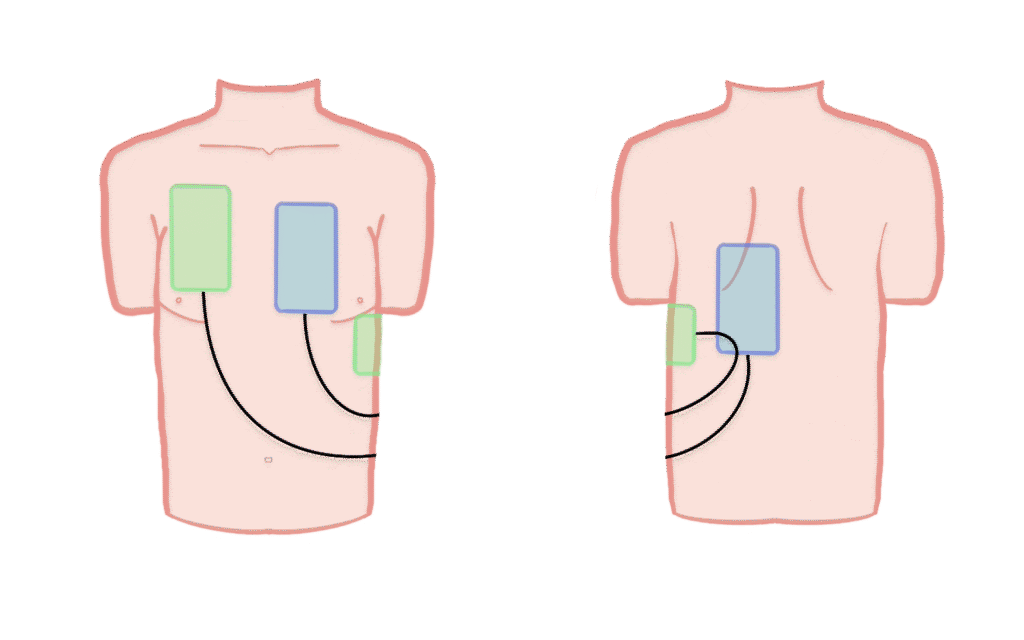
Intervention group
Patients randomized to the intervention group will receive Double Sequential Defibrillation (DSD). A second defibrillator is applied in the A-P position, and if VT/VF persists, shocks are delivered sequentially. All further defibrillations follow the DSD strategy until ROSC, termination of resuscitation, or transport to hospital.
Flowchart